On this page:
- Introduction
- Chimeric Antigen Receptor T-Cell Therapy: How it Works
- Watch the CAR T-Cell Therapy Process in Augmented Reality
- FDA Approved Treatments
- Dr. Brentjens Talks about CAR T-Cell Therapy
- Possible Side Effects of CAR T-Cell Therapy
- Results, Limitations, and the Future of CAR T-Cell Therapy
- Video about CAR T-Cell Therapy
- Enrolling in a Trial
- Sponsors & Supporters
Introduction
The immune system is the body’s defense against infection and cancer. It is made up of billions of cells that are divided into several different types.
Lymphocytes, a subtype of white blood cells, comprise a major portion of the immune system. There are three types of lymphocytes
- B lymphocytes (B cells) make antibodies to fight infection.
- T lymphocytes (T cells) have several functions, including helping B lymphocytes to make antibodies to fight infection, and directly killing infected cells in the body.
- Natural killer cells also attack infected cells and eliminate viruses.
Immunotherapy
- Is a type of treatment that utilizes the body’s own immune system to fight cancer
- Improves the body’s ability to detect and kill cancer cells
- Is based on the concept that immune cells or antibodies can recognize and kill cancer cells.
Immune cells or antibodies can be produced in the laboratory under tightly controlled conditions and then given to patients to treat cancer. Several types of immunotherapy are either approved for use or are under study in clinical trials to determine their effectiveness in treating various types of cancer.
Chimeric Antigen Receptor T-Cell Therapy: How it Works
Watch in Augmented Reality
Use your smartphone, tablet, or other mobile device to see the CAR T-cell therapy process in action.
Download the How CAR T-Cell Therapy Works app on your mobile device:
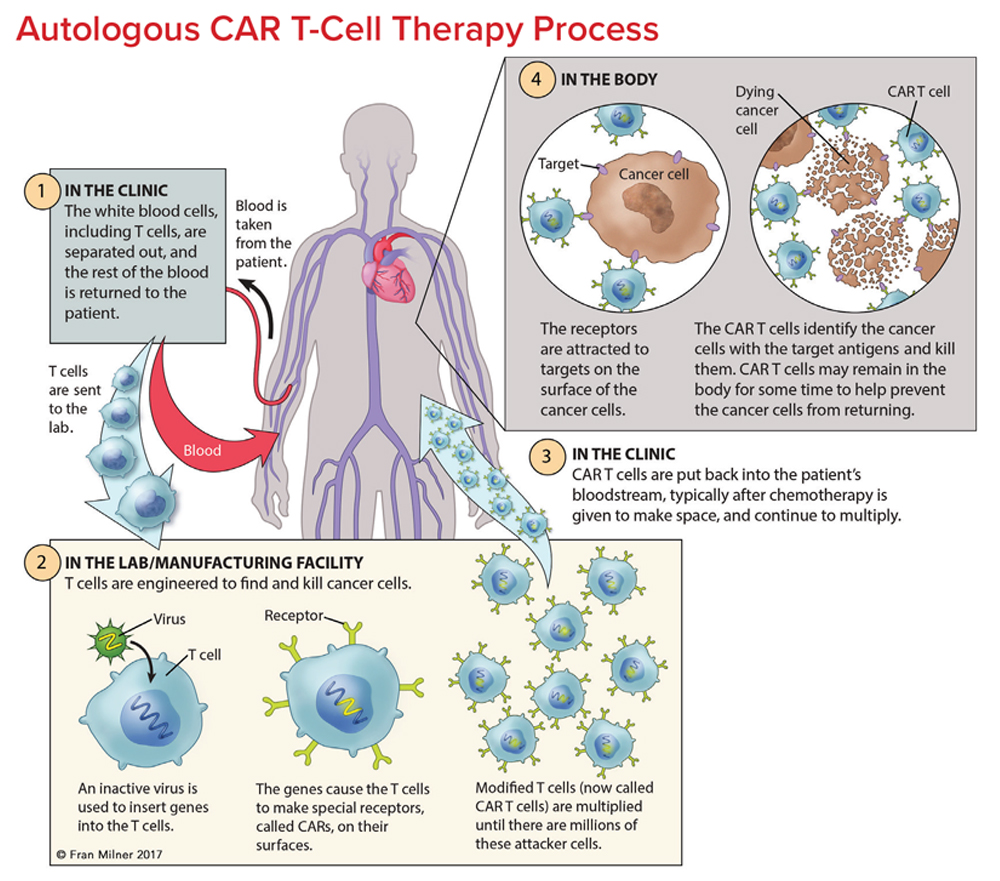
T cells are collected from a patient. T cells are collected via apheresis, a procedure during which blood is withdrawn from the body and one or more blood components (such as plasma, platelets or white blood cells) are removed. The remaining blood is then returned to the body.
T cells are reengineered in a laboratory. The T cells are sent to a laboratory or a drug manufacturing facility where they are genetically engineered, by introducing DNA into them, to produce chimeric antigen receptors (CARs) on the surface of the cells.
After this reengineering, the T cells are known as “chimeric antigen receptor (CAR) T cells.” CARs are proteins that allow the T cells to recognize an antigen on targeted tumor cells.
The reengineered CAR T cells are then multiplied. The number of the patient’s genetically modified T cells is “expanded” by growing cells in the laboratory. When there are enough of them, these CAR T cells are frozen and sent to the hospital or center where the patient is being treated.
At the hospital or treatment center, the CAR T cells are thawed and then infused into the patient. Many patients are given a brief course of one or more chemotherapy agents, called “lymphodepletion,” before they receive the infusion of CAR T cells. CAR T cells that have been returned to the patient’s bloodstream multiply in number. These are the “attacker” cells that will recognize, and attack, cells that have the targeted antigen on their surface.
The CAR T cells may help guard against recurrence. CAR T cells may eradicate all of the cancer cells and may remain in the body months after the infusion has been completed. The therapy has resulted in long-term remissions for some types of blood cancer.
FDA Approved Treatments
- Axicabtagene ciloleucel (YescartaTM) is FDA approved for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. Yescarta is also indicated for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy. Yescarta is not indicated for the treatment of patients with primary central nervous system lymphoma. It is a CD19-directed genetically modified autologous T cell immunotherapy. You can read more about this drug here.
- Brexucabtagene autoleucel (Tecartus®) is FDA approved for for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
This indication is approved under accelerated approval based on overall response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. You can read more about this drug here. - Idecabtagene vicleucel (Abecma®) is FDA approved for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. You can read more about this drug here.
- Tisagenlecleucel (KymriahTM) is FDA approved for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse. Kymriah is also approved for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma. Limitation of Use: Kymriah is not indicated for treatment of patients with primary central nervous system lymphoma. It is a CD19-directed genetically modified autologous T cell immunotherapy. You can read more about this drug here.
- Tocilizumab (Actemra®) is approved for the treatment of adults and pediatric patients 2 years of age and older with chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome (CRS). You can read more about this drug here.
CAR T-cell therapy continues to be available to patients who are participating in a clinical trial. Trial protocols vary. Depending on the clinical trial, care may be provided in either a hospital setting or an intensive outpatient treatment center with experience administering cellular immunotherapy. Patients may have to stay at the treatment facility and may need to plan to stay close by before, during or following treatment. Some trial protocols require patients to confirm the availability of a caregiver before they can enroll in the trial.

Possible Side Effects of CAR T-Cell Therapy
Cytokine-Release Syndrome (CRS). This potentially serious side effect is frequently associated with CAR T-cell therapy. Cytokines (chemical messengers that help the T cells carry out their functions) are produced when the CAR T cells multiple in the body and kill the cancer cells. CRS symptoms can range from mild flulike symptoms that include
- Nausea
- Fatigue
- Headache
- Chills
- Fever
The symptoms of CRS can also be more serious such as
- Low blood pressure
- Tachycardia (abnormally rapid heart rate)
- Capillary leakage (fluid and proteins leaking out of tiny blood vessels and flowing into surrounding tissues, resulting in dangerously low blood pressure)
- Cardiac arrest
- Cardiac arrhythmias
- Cardiac failure
- Hemophagocytic lymphohistiocytosis (life-threatening immune system activation)/macrophage activation syndrome (life-threatening activation of macrophages) (HLH/MAS)
- Hypoxia (lack of oxygen reaching the tissue)
- Renal insufficiency (poor function of the kidneys)
- Poor lung oxygenation
- Multiple organ failure
- Neurological symptoms (see below)
Severe CRS requires intensive care treatment. Although most symptoms are reversible, the potential life-threatening risk of CAR T-cell therapy should not be underestimated. Deaths have been reported in CAR-T cell therapy trials.
Depending on the patient and the CAR T cells, CRS may occur within 1 to 21 days of CAR T-cell infusion. The duration of CRS is variable and it depends on the type of intervention used to manage it.
Macrophage Activation Syndrome (MAS). This side effect is closely associated with severe CRS. This syndrome is a condition caused by the excessive activation and multiplication of T cells and macrophages. It is generally seen in patients with chronic autoimmune and rheumatic diseases. Fortunately, research has shown that MAS (like CRS) can be mitigated by the infusion of the monoclonal antibody tocilizumab (Actemra®). Corticosteroids and anticytokine therapy can be considered as treatment options if MAS is severe and the symptoms persist or are getting worse.
Neurologic Toxicities. The frequency, severity and nature of neurological effects appear different between CAR-T products. Common symptoms include language impairment (aphasia), confusion, delirium, involuntary muscle twitching, hallucinations, or unresponsiveness. Seizures have also been reported.
Neurotoxicity has been reversible in most cases and the symptoms have resolved over several days without intervention or apparent long-term effects. However there can be life-threatening adverse neurological events. The cause of neurotoxicity is the subject of intense investigation by researchers.
Tumor Lysis Syndrome (TLS). Another known side effect of CAR T-cell therapy is tumor lysis syndrome (TLS), a group of metabolic complications that can occur due to the breakdown of dying cells—usually at the onset of toxic cancer treatments. However, TLS can be delayed and may occur one month or more after CAR T-cell therapy. TLS can cause organ damage and can be a life-threatening complication of any treatment that causes breakdown of cancer cells, including CAR T cells. The complication has been managed by standard supportive therapy.
Anaphylaxis (Life-threatening Allergic Reaction). There is potential for a patient receiving CAR T-cell therapy to have an overwhelming immune response against the CAR itself, called “anaphylaxis.” Symptoms associated with anaphylaxis include hives, facial swelling, low blood pressure and respiratory distress. There have been a few reports of acute anaphylaxis. Thorough monitoring and immediate treatment of this life-threatening side effect are critical for patients receiving CAR T-cell therapy.
On-target, Off-tumor Toxicity. An important factor in the safe and successful use of CAR T cells is choosing the proper tumor-associated antigen to target. Unfortunately, it is rare to find such an ideal target. Many tumor antigens are also expressed on healthy cells in tissues. Damage to such noncancerous normal tissue by CAR T cells may pose life-threatening risks, especially when cells in essential tissues such as the heart, lung or liver express the target antigen.
B-Cell Aplasia. CAR T-cell therapy targeting antigens found on the surface of B cells not only destroys cancerous B cells but also normal B cells. Therefore, B cell aplasia (low numbers of B cells or absent B cells) is an expected result of successful CD19-specific CAR T-cell treatment and has served as a useful indicator of ongoing CAR T-cell activity. This effect results in less ability to make the antibodies that protect against infection. Intravenous or subcutaneous immunoglobulin replacement therapy may be given with the aim of preventing infection. Long-term follow-up study is needed to assess the late effects of B-cell aplasia.
Results, Limitations, and the Future of CAR T-Cell Therapy
Chimeric antigen receptor T-cell clinical trials have generated impressive results in the early outcomes of CAR T-cell therapy patients with blood cancers.
In some studies, up to 90 percent of children and adults with B-ALL whose disease had either relapsed multiple times, or failed to respond to standard therapies, achieved remission after receiving CAR T-cell therapy. Relapses may be due to the tumor cells losing the expression of the cluster of differentiation (CD-19) antigen, the limited persistence of CAR T cells, or inhibition of CAR T-cell activity.
Studies of CAR T-cell therapy in other blood cancers, including chronic lymphocytic leukemia (CLL), as well as multiple myeloma, also show potential. Research is also under way, exploring the application of CAR T-cell therapy in the treatment of solid tumors.
Most patients participating in CAR T-cell trials have only been followed for a relatively short time; however, data providing information about early responses to therapy is fast emerging. Researchers will be able to predict the duration of these responses after trial participants have been followed over the long term. It is important for more pediatric and adult patients to be enrolled in clinical trials. Larger study samples, evaluated over more extended periods, will help researchers further understand the impact of this type of therapy, ways to reduce its toxicity and improve the management of adverse side effects.
Video about CAR T-Cell Therapy

Enrolling in a Trial
Many CAR-T cell therapies are being studied in clinical trials. Talk with your doctor about whether participation in a CAR T-cell therapy clinical trial is an option for you. Obtaining another opinion from a hematologist-oncologist (a blood cancer specialist), may be helpful in finding additional clinical-trial information as well. When you and your doctor discuss CAR T-cell therapy as a potential treatment option for you, it may be helpful to have
- A list of questions to ask concerning risks versus benefits of such a trial (click here for lists of suggested questions).
- A family member, friend, or another advocate with you for support and to take notes.
Clinical Trial Support Center
Work one-on-one with an LLS Clinical Trial Nurse Navigator who will help you find clinical trials and personally assist you throughout the entire clinical-trial process.
For more information or to contact us, click here.
Related Links
- Contact an LLS Information Specialist for more information
- Download or order this free one-page graphic description of The CAR T-Cell Therapy Process.
- Download or order this free fact sheet, Chimeric Antigen Receptor (CAR) T-Cell Therapy Facts.




