Bringing Precision Medicine to AML Patients
LLS’S BEAT AML® MASTER CLINICAL TRIAL
Going on the Offensive against Acute Myeloid Leukemia
Acute myeloid leukemia (AML) is one of the most lethal blood cancers that takes more than 10,000 lives in the U.S. each year.
While therapies for other blood cancers have made remarkable leaps forward, the standard of care for AML – a combination of toxic chemotherapies – has changed very little over the past four decades. Until now.
Advancements in genomics and precision medicine are fueling a renaissance in AML research.
Today, we know that AML is not a single disease, but rather a group of more than 10 subtypes and other rare mutations. Once an elusive enemy, researchers are now able to identify and target these specific subtypes, and nine new therapies – all advanced with LLS support – have been added to our arsenal in the past two years, following approval from the U.S. Food and Drug Administration (FDA).
Still, much work remains. Among adults over the age of 60, only about one in four AML patients survives five years after diagnosis. LLS is leading the charge against AML through the Beat AML® Master Clinical Trial, a collaborative clinical trial that aims to change the paradigm of treatment through a precision medicine approach.
How is the Beat AML® Master Clinical Trial transforming AML treatment?
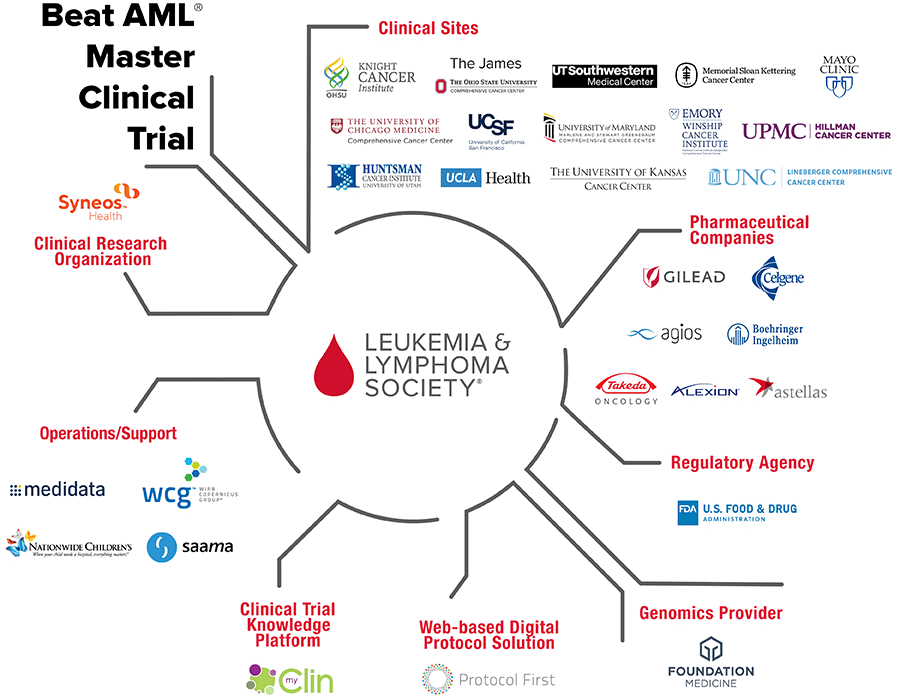
The Beat AML® Master Clinical Trial is the first collaborative precision medicine clinical trial in a blood cancer. The trial uses advanced genomic technology to identify each patient’s cancer-driving genetic mutations, and then matches patients to the most promising, targeted treatment. While a typical clinical trial studies one drug or one combination of drugs, we are testing multiple therapies in multiple study arms simultaneously. This Master Trial protocol – developed with guidance from the FDA – not only has the power to bring new therapies to AML patients faster, but also has the potential to stand as a model for future clinical trials for other cancers.

LLS is leading the charge against AML
LLS has brought together the best and brightest minds across the cancer ecosystem, including three world-renowned scientists who are leading the trial: Brian Druker, MD, Director, The Knight Cancer Institute at Oregon Health & Science University; John C. Byrd, MD, D. Warren Brown Chair of Leukemia Research of The Ohio State University Comprehensive Cancer Center; and Ross L Levine, MD, Director of the Center for Hematologic Malignancies at Memorial Sloan Kettering Cancer Center. Additionally, multiple pharmaceutical companies, prominent scientists at top cancer centers, several technology companies and the U.S. Food and Drug Administration join us in a shared commitment to bring the promise of precision medicine to AML patients.
Why is the Beat AML® Master Clinical Trial so groundbreaking?
To bring new and better treatments to AML patients in urgent need, we need to think and act boldly.
A FIRST FOR LLS:
Given our leadership in AML research – one-quarter of our annual research funding is dedicated to AML – as well as our commitment to putting patients at the forefront, LLS is uniquely qualified to drive this powerful collaboration. In fact, LLS is the first nonprofit health organization to sponsor a cancer clinical trial.
A FOCUS ON NEWLY DIAGNOSED PATIENTS:
Most AML clinical trials center on patients who have relapsed or not responded to other treatments. This trial focuses on newly diagnosed, untreated patients aged 60 or older – allowing researchers to identify genetic mutations early and offering a better chance for successful treatment.
AN UNPRECEDENTED GENOMIC SCREENING TURNAROUND:
Because AML progresses so quickly, patients need to be treated based on their particular subtype right away. For this trial, advanced genomic screening is being completed within seven days, an unparalleled timeframe.
What has the Beat AML® Master Clinical Trial achieved?
The Beat AML® Master Clinical Trial continues to exceed expectations.
The trial is currently enrolling patients at 16 prestigious cancer centers across the country and more pharmaceutical companies continue to join the collaboration. To learn more contact the LLS Information Resource Center: 800-955-4572.