
More than three years after the first chimeric antigen receptor (CAR) T-cell therapy achieved U.S. Food and Drug Administration (FDA) approval, the revolutionary approach that has upended blood cancer treatment continues to generate excitement.
LLS support over more than two decades helped lay the groundwork for this approach to therapy that has been a game-changer for patients who have relapsed multiple times and seemingly exhausted their treatment options. The method works by infusing immune (T) cells with cancer-attacking synthetic receptors that seek out and bind to a specific protein on the surface of the cancer cells, causing their destruction. The CAR-T cells continue to expand and fight the cancer cells, becoming a living drug within the patient’s body. Pediatric acute lymphoblastic leukemia patients and adults with large B-cell lymphomas have benefited from long-lasting remissions from the first CAR-Ts approved. The latest CAR-T approval was in August for patients with mantle cell lymphoma.
Consensus is building that the next CAR-Ts on the horizon will be for multiple myeloma patients and follicular lymphoma and marginal zone lymphoma patients who have relapsed multiple times. During this week’s virtual 62nd American Society of Hematology (ASH) annual meeting, a slew of presentations makes clear several of these are likely to get approved in early 2021.
The myeloma CAR-Ts all target a protein called BCMA found on cancerous plasma cells in myeloma patients. BCMA has emerged as a prime target for myeloma treatment, and the first BCMA-targeting therapy, an antibody-drug conjugate (an antibody bound to a cytotoxic agent), was approved by the FDA in August – read more here.
Of the multiple companies vying in the BCMA-targeting CAR-T space, Johnson & Johnson’s product, ciltacabtagene autoleucel (cilta-cel), appears to be the frontrunner, with the highest response rates and longest-lasting CAR-T cells. In a study of 97 patients, 97 percent responded to the treatment, and 67 percent had a complete response, meaning no cancer cells were detectable. At just over one year (the median follow-up, meaning at least 50 percent of the patients had been on the trial), 77 percent of the patients were still alive and their cancer had not gotten worse.
Johnson & Johnson plans to submit its data to the FDA within the next few weeks. What's more, Bristol Myers Squibb/Bluebird’s idecabtagene vicleucel (ide-cel) – also promising while not as long-lasting in trials to date – is already before the FDA with a decision anticipated in March. While not involved in the study present at the ASH meeting, an LLS-funded researcher at Emory University, Madhav Dhokapkar, MBBS, is studying this therapy to find ways to make it more durable, safer, and more effective. Many CAR-T products from Chinese companies were also presented at the meeting.
The length of time that BCMA-targeting CAR-T cells can last in patients has been questionable, so the results presented at this ASH meeting are encouraging. At the same time, the deaths of several patients on trials have raised concerns about potential toxicities, including dangerous neurological side effects and another extreme immune response called cytokine release syndrome. Despite this, the FDA might determine that the benefits for patients without other treatment options outweigh the risks.
Patients with follicular (FL) and marginal zone lymphoma (MZL), two slow-moving “indolent” forms of non-Hodgkin lymphoma, also have reason for hope. Results from a clinical trial entitled ZUMA-5, testing a CAR-T developed by Kite Pharma, a Gilead company, showed high response rates for patients with both forms of the disease. LLS helped Kite Pharma launch their CAR-T program through a partnership entered in 2015.
Of 104 patients evaluated, 84 with FL and 20 with MZL, 92 percent had an objective response, meaning their cancer cells were reduced; 76 percent of patients had a complete response, meaning the cancer became undetectable after a median follow-up of 17.5 months (the time when 50 percent of the patients have been on trial).
Researchers across the globe – with support from LLS – continue to work tirelessly to usher in the next generation of CAR T-cell therapies so that they're safer and more efficacious for more patients with blood cancers.
Bispecific Antibodies – A Different Way to Harness the Boost the Body’s Immune System
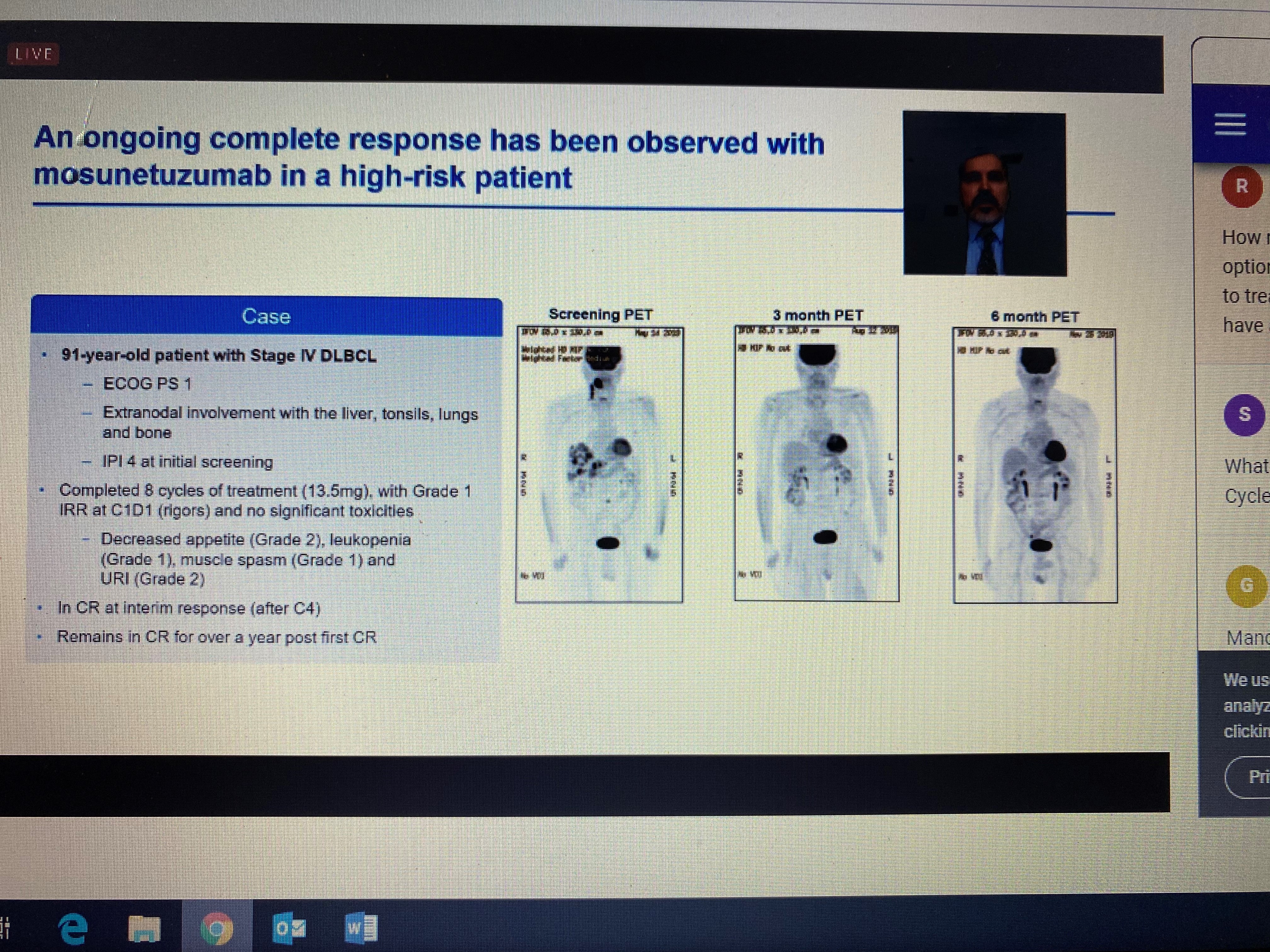
Several studies also highlighted the promise of bispecific antibodies, which are designed to bind two proteins: one on the surface of tumor cells (CD20) and the other on the surface of the recipient’s T cells (CD3). The studies were in patients with lymphoma who were either too old or sick to endure standard chemotherapy treatment, or whose previous treatments had failed, including CAR-T in some cases. The results were impressive, with high, durable response rates. There are advantages and disadvantages of this treatment approach as compared to CAR-T. Manufacturing CAR-T is a long, complicated, and costly process requiring the extraction of the patient’s own cells. Bispecific antibodies use donor cells and can be manufactured as an “off-the-shelf” product, so the manufacturing and wait time is far less. On the other hand, CAR-T is a one-time infusion, while bispecific antibodies have to be administered to the patient repeatedly for as long as the patient is responding to the treatment. Some of the versions presented at the meeting can be given by an injection under the skin (subcutaneous) as opposed to intravenous infusion, requiring less time in the clinic and making the treatment regimen somewhat less onerous for the patient. While promising, the results for these therapies are still early so we will be keeping an eye on these advancements.
Visit this blog again for ongoing coverage from #ASH20, and click here to read the previous blog from ASH.
