Since 2017, three TAP-supported therapies have been approved by the U.S. Food and Drug Administration (FDA):
- CPX-351 (Vyxeos®), first approved treatment (an innovative reformulation of two chemotherapies) for patients with certain types of high-risk acute myeloid leukemia (AML) - approved on August 3 2017 with clinical data published in J Clin Oncol
- Axicabtagene ciloleucel (Yescarta®), first CAR T-cell immunotherapy approved for patients with non-Hodgkin lymphoma (NHL) and transformed follicular lymphoma (tFL) - approved on October 18 2017 with clinical data published in Lancet Oncol
- Tagraxofusp-erzs (Elzonris®), first approved therapy for children and adults with blastic plasmacytoid dendritic cell neoplasm (BPDCN) - approved on December 21 2018 with clinical data published in N Engl J Med
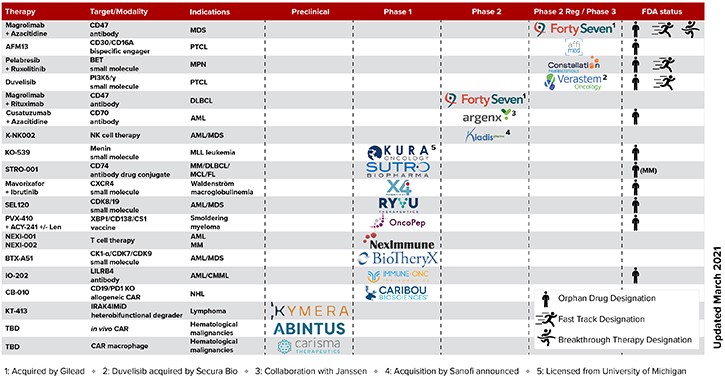
Currently, there are over 20 TAP-supported companies or institutions with assets in active development, including 5 ongoing or planned registration-enabling clinical studies.